Synthesis of Natural Products and Synthetic Methodologies
Research in the Magnus Lab is focused on the total synthesis of natural products. Alkaloids, terepenoids, ketolides, and other classes of natural products have all been completed in our labs. All of the current projects involve the development of new concise strategies and this, in course, frequently leads to the discovery of new reactions. These projects are summarized below.
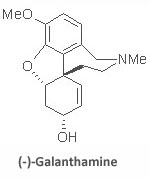
Total Synthesis of (-)-Galanthamine: The amaryllidaceae alkaloid (-)-galanthamine is a potent acetylcholinesterase inhibitor and has found extensive use in the early treatment of Alzheimer’s disease. This has caused a renewed interest in its synthesis, since its extraction from the bulbs of snow drops does not supply sufficient material for the clinical evaluation of early Alzheimer’s patients. Currently, commercial supplies of galanthamine are available from Sanochemia AG using the Frohlich-Jordis route in nine steps and an overall yield of 12%.
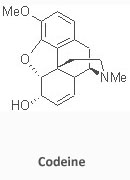
Total Synthesis of Codeine: Since the report by Robinson in 1925 of the structures of codeine and morphine there have been numerous total and formal syntheses of these alkaloids spanning a period of approximately 55 years from 1952 to 2007. Nevertheless, there is no practical synthetic source of these opium alkaloids other than the Rice adaptation of the Grewe strategy.
(pdf click here) C&EN coverage (click here)
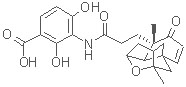
Total Synthesis of Platensimycin: Discovered in 2006 by scientists in the Merck Research Laboratories, platensimycin is the first member of a new class of antibiotics. Platensimycin exhibits potent antibiotic properties toward gram-positive pathogens, working by inhibition of bacterial fatty acid biosynthesis.
(pdf click here)
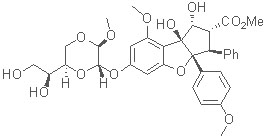
Total Synthesis of Silvestrol: Isolated in 2004 from the twigs and fruits of Aglaia foveolata (Pannell), Silvestrol shows cytotoxic activity comparable to Taxol. No total synthesis of silvestrol has been published to date, but there have been numerous syntheses of the parent rocaglamide and the 1,4-dioxanyloxy fragment. Our strategy uses a Nazarov cyclization to form the cyclopenta[b]benzofuran skeleton.
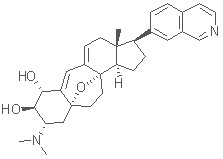
Total Synthesis of Cortistatin A: Cortistatin A is one of eleven anti-angiogenic steroidal alkaloids which were recently isolated from the marine sponge Corticium simplex. In preliminary screenings, cortistatin A exhibited highly selective anti-proliferative activity against human umbilical vein endothelial cells (HUVECs), and is thus a promising anti-tumor drug candidate. (pdf click here)
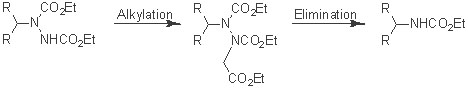
N,N Bond Cleavage Methodology: Traditional methods that are used to cleave the N-N bond
in N,N -alkyl(aryl), N,N -monoacyl, diacyl, and triacyl derivatives
involve exposure of these compounds to classical
reduction conditions such as Raney nickel, Na/NH3, Li/NH3, SmI2, B2H6, NiCl2·2H2O-LiDTBB (cat.), and
R3Si-H. Oxidative cleavage of the N-N bond has also
been reported, and there are also sporadic reports of
eliminative methods to rupture N-N bonds. There remains a need for a practical, anionic N-N bond cleavage method for converting hydrazino compounds into amine derivatives. (pdf click here)